Sickle Cell Disease Flashcards
What does the definition of Sickle cell disease refers to?
What is meant by the term Sickle Cell Anemia?
Sickle cell disease is a group of genetic disorders in which a patient inherits two abnormal beta globin genes, one from each parent, with at least one of the abnormal genes coding for the sickle hemoglobin variant.
Sickle cell disease also occurs if the sickle mutation is co-inherited with another variant beta globin (e.g. Hb C) or with a beta thalassemia mutation causing decreased production of normal beta globin.
If the patient is homozygous for he sickle cell mutation (Hb SS), the disease is called “sickle cell anemia”.

Where can you find the Hb S allele most commonly?
Africa, India, and the Mediterranean
Describe how sickling occur?
What factors lead to increase sickling?
What factors decrease sickling?
Normal adult hemoglobin is soluble in aqueous solution. In contrast, sickle hemoglobin polymerizes into 14-strand helical fibers and precipitates within the red blood cell under deoxygenated conditions (figure to right). This distorts the shape of the red blood cell causing it to assume a “sickle form”. When reoxygenated, the sickle polymers dissolve and the red blood cell returns to its normal shape. The decreased deformability of the sickled cells causes damage to the red cell membrane and after several deoxygenation-reoxygenation cycles, the cell becomes irreversibly sickled and is lysed (destroyed).
Factors that lead to sickling:
Decreased O2, pH, dehydration (loss of RBC volume), and decreased hemoglobin (since less O2 can be carried).
Factors that decrease sickling:
Hb F (since it has a high affinity for O2 and it is incorporated into the polymer, which prevents sickling), increased Hb A (Hb A is not incorporated into the fiber and slows polymerization due to dilution of Hb S).

Describe why the RCB in sickle cell disease can cause vasoocclusion?

Even when not sickled, the red blood cell in sickle cell disease is “sticky” due to membrane injury and retention of adhesion molecules on the surface of the RBC.
–>This results in adhesion of sickle RBCs in the microvascular circulation which can cause transient vaso-occlusion (partial or total blockage of blood flow through the vessel), vessel wall injury and endothelial remodeling. Narrowing of these vessels and chronic organ damage can occur due to slow or absent blood flow through this microcirculation.

Dscribe chronic hemolytic anemia seen in sickle cell disease?
How long does the sickle RBC survives?
In terms of laboratory findings:
1) Reticulocyte count
2) WBC and platelet
3) RDW
4) Cells seen in peripheral smears
5) Chemistry profile
The sickle RBC is rigid and fragile, resulting in chronic RBC destruction (hemolytic anemia). In homozygous sickle cell anemia (Hb SS), the sickle RBC survives for approximately 20 days in the circulation compared to 120 days for normal RBCs. This results in characteristic changes in laboratory tests including:
1- Anemia with compensatory increase in reticulocyte count – the severity of the anemia varies with the type of sickle cell disease.
2- Increased baseline white blood cell count and platelet count – due to exuberant bone marrow response to hemolytic anemia and other factors. Increased baseline WBC count has been associated with increased mortality and morbidity in patients with sickle cell anemia.
3- Increased RDW – because sickle RBCs transition from sickled to unsickled, changing shape, and because reticulocytes are very young, large RBCs, there is significant variation in the size of RBCs as analyzed by automated cell counters
4- Abnormal peripheral smear – sickle forms, schistocytes (“broken”, irregular cells), polychromasia (blue-colored cells representing reticulocytes), anisocytosis (variation in size of RBCs), **poikilocytes (variation in shape of RBCs), Howell-Jolly bodies (small purple dots of remnant DNA in patients without a functional spleen), target cells and Hb C crystals (red “rods” within RBCs) are seen in Hb SC disease, microcytosis and target cells are seen in sickle thalassemia diseases.
Abnormal chemistry profile – increased total/indirect bilirubin, lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) as they are released from lysed RBCs.

Just know the possible outcomes of chronic hemolytic anemia in sickle cell disease patients?
Aplastic crisis –Since sickle cell disease patients rely on an increased reticulocyte count to compensate for increased RBC destruction, anything that compromises the bone marrow’s ability to rapidly produce RBCs can result in a sudden drop in hemoglobin, called an aplastic crisis. The characteristic finding is a low reticulocyte count and severe anemia. In children, an important cause of aplastic crisis is Parvovirus B19, which causes “fifth disease” and infects RBC precursors, arresting their development into mature RBCs. This infection is usually transient, but patients may require RBC transfusions if the hemoglobin falls significantly. Other severe infections, medications or vitamin (e.g. folic acid) deficiencies can also result in an aplastic crisis.
Growth retardation/delay – May be multifactorial and related to anemia, increased metabolic rate due to increased RBC production and vitamin deficiencies
Bilirubin gallstones – Chronic elevation in bilirubin due to hemolytic anemia can lead to formation of gallstones. This usually occurs by the second decade of life.
Explain how adhesion and vaso-occlusion of sickel RBCs damages the body:
What is a pain crisis?
Dactylitis?
Acute chest syndrome?
Priapism?
Bone infarction?
Acute RBC adhesion/vaso-occlusion
In the setting of hypoxia, dehydration, inflammation, infection or other stresses, not only are RBCs more likely to sickle but blood vessels can become acutely damaged and constricted, which may promote significant sudden vaso-occlusion. This results in a “pain crisis”, in which acute severe pain develops relatively rapidly in a pattern that is unique to each patient and may involve any part of the body, most commonly in the arms, legs, chest or abdomen. The pain is likely due to many factors including reversible ischemia, and resolves as the inciting factors (e.g. hypoxia, dehydration) are improved. During some pain crises, acute severe vaso-occlusion may occur in critical organs causing acute end-organ injury.
Other significant acute vaso-occlusive complications include:
Dactylitis – usually seen in infants with sickle cell anemia and Hb S-β0 thalassemia in which self-limited acute severe swelling of the hands and feet can occur and may be one of the earliest manifestations of sickle cell disease
Acute chest syndrome – sickle RBCs can become trapped in the lung circulation, which damages the vessel lining (endothelium), promoting fluid to leak into the lungs, compromising the ability to oxygenate the blood. The patient may develop severe chest pain, fever, low oxygen saturation, and experience a fall in hemoglobin due to the trapping of RBCs in the lung. This is one of the most common acute causes of death in sickle cell disease and may be triggered by pneumonia or embolization of fat from the bone marrow.
Acute multi-organ failure – In some patients, severe acute renal failure and liver failure accompany acute chest syndrome
Priapism – sickle RBCs can be trapped in the penis, with obstruction of outflow, resulting in sustained painful erections. Unless treated, these can result in impotence.
Bone infarction – Focal areas of bone may sustain enough ischemia to become permanently damaged or necrotic. These areas can remain painful for extended periods of time and may become a source of chronic pain or a site of osteomyelitis (bone infection).
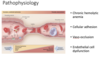
What are the chnronic consequences of RBC adhesion/vaso-occlusion?
Chronic RBC adhesion/vaso-occlusion
Chronic vaso-occlusion and endothelial cell damage can cause end-organ damage to nearly every system in the body. The most commonly affected organ systems include the following:
Spleen – The microcirculation of the spleen is especially susceptible to occlusion and injury by sickle RBCs. When large numbers of sickle RBCs become abruptly trapped in the microcirculation of the spleen, severe anemia and circulatory shock can occur, a complication called splenic sequestration. Even if overt evidence of sequestration isn’t seen, nearly all patients with sickle cell anemia (Hb SS) chronically occlude the spleen’s microcirculation, resulting in “auto-infarction” (destruction) of the spleen by the age of 5 years. This process begins during the first year of life and slowly compromises the spleens ability to kill encapsulated organisms (e.g. pneumococcus, meningococcus, hemophilus). Sepsis (overwhelming blood infection) with these organisms is a common cause of death for infants and young children with sickle cell disease. The incidence of sepsis is reduced with the use of prophylactic (preventative) penicillin and prompt treatment of fevers with additional antibiotic therapy. The early institution of penicillin prophylaxis is the rationale for newborn screening for sickle cell disease.
Central Nervous System – The large blood vessels of the central nervous system can be significantly damaged by sickle RBCs. Up to 10% of children with sickle cell anemia (Hb SS) experience an overt large vessel stroke due to this chronic injury, and a larger percentage experience learning disabilities and more subtle neurologic problems. An increase in the velocity of blood flow through the middle cerebral artery, as detected by transcutaneous Doppler, can predict which children are at increased risk of stroke. This risk can be reduced by prophylactic blood transfusions. Adults with sickle cell disease are more likely to have hemorrhages from progressive weakening and rupture of these vessels.
Lung – The microcirculation of the lung is vulnerable to damage from sickle RBCs. Damage to these vessels makes ti harder for blood to flow through the lungs, resulting in an increased pressure in the pulmonary arteries, a condition called pulmonary arterial hypertension (PAH). PAH, which puts strain on the right side of the heart (cor pulmonale), may affect 30-40% of patients with sickle cell disease and is now one of the most common chronic causes of death in adults with sickle cell disease.
Kidney – The tubules of the kidney are damaged by chronic vaso-occlusion, resulting in the inability to concentrate the urine to avoid dehydration. Hematuria (blood in the urine) may occur due to ischemia to the collecting system (papillary necrosis), which may also cause severe flank pain. The glomerulus may be affected, most commonly initially manifested by an enlargement of the glomerulus and protein in the urine. Ultimately, up to 10% of adult sickle cell patients will develop renal insufficiency due to permanent damage and scarring of the glomerulus (focal segmental glomerular sclerosis), and some will require dialysis and/or kidney transplantation.
Retina – The retinal vessels are subject to chronic injury, resulting in the propensity for abnormal vessel formation and hemorrhage, which can lead to retinal detachment and blindness
Other areas – The femoral heads may develop avascular necrosis, a source of chronic pain and progressive joint deterioration, leading to hip and shoulder replacement. Skin ulcers, likely due to microvascular ischemia and poor wound healing may form around the ankles.
What can be observed from laboratory findings?
What is the evidence of hemolysis?
Complete blood count shows:
- Varying degrees of anemia
- May be microcytic or normocytic depending on genotype
-Reticulocyte count increased (due to short lifespan of sickle RBCs)
- Peripheral Smear – sickle cells, Howell-Jolly bodies, nucleated red cells (this happens because the bone marrow is trying to produce a lot of RBCS, and can mistakenly send premature reticulocytes into the circulation), polychromasia (blue color cells representing reticulocytes).
- Evidence of hemolysis:
- Increased LDH and unconjugated bilirubin, decreased haptoglobin



What is the Sickle Solubility Testing?
Can it distinguish sickle trait from disease?
Can it distinguish type of sickle cell?
•Detects the sickle hemoglobin in concentrations as low as 8-20%
•CANNOT distinguish sickle trait from disease
•CANNOT distinguish type of sickle cell disease
Hemoglobin separation results:

Epidemiology

Sickle Cell Trait (Heterozygotes, Carrier state)

Acute complications:
What is Vaso-Occlusive pain crisis? Where is it usually located?
What causes it?
What is the treatment?
This refers to acute onset of severe pain that may be located in the extremeties, chest, back, and abdomen.
In the setting of hypoxia, dehydration, inflammation, infection or other stresses, not only are RBCs more likely to sickle but blood vessels can become acutely damaged and constricted, which may promote significant sudden vaso-occlusion. This results in a “pain crisis”, in which acute severe pain develops relatively rapidly in a pattern that is unique to each patient and may involve any part of the body, most commonly in the arms, legs, chest or abdomen. The pain is likely due to many factors including reversible ischemia, and resolves as the inciting factors (e.g. hypoxia, dehydration) are improved.
Management:
NSAIDs and opiates (individualized, outpatient or inpatient), nonpharmacologic adjuncts (heat therapy, relaxation, massage, hydration)