Exam 1 Flashcards
Describe the process of Red Cell Measurements using aperture impedance:
Physiologic variables that affect one’s RBC s include age, sex, and altitude. Clinical laboratories determine their pediatric and adult (male and female) reference ranges , which are relevant to the population served by the laboratory. More than the expected number of red cells for one’s age and gender is referred to as erythrocytosis (sometimes polycythemia in the context of a neoplastic expansion of red cell mass). Red cells can be counted by aperture impedance, light scattering techniques or a combination.
Aperture impedance was patented by Wallace Coulter this is also known as the Coulter principle. It can also be used to count RBCs, WBCs, and platelets.
By this method electrical current is established across an aperture of known dimensions. When a cell or particle passes through the aperture, the cell or particle changes the current flow and causes a voltage surge. The voltage, first rising above and then falling below a certain threshold, indicates that a cell or particle has passed through the sensing zone. Cells and particles are counted by monitoring the number of voltage pulses that occur during a specified measurement interval.
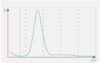
The volume of the cell can be determined by measuring the magnitude of the pulse generated. Thresholds or electronic “ gates ” are set, allowing the analyzer to discriminate RBC pulses from smaller platelet pulses, debris and electrical noise. As seen above, this technique produces a histogram of events with the range of pulse magnitude s on the x - axis and the number of events on the y - axis.
How does the Sysmex method for measuring Hgb works?

Cathelicidin bind to what ?
negatively-charged pathogen membranes, in which they insert themselves and make pores
Neutrophils
- Mature neutrophil cytoplasm is acidophilic with fine granules.
- The nucleus has clumped chromatin divided into two to five distinct lobes linked by filaments.
- Too few neutrophils is termed neutropenia, while too many is neutrophilia. In adults neutrophils are the ***most abundant white cell in the peripheral blood.
STEP:
These are the acute inflammatory response cells, which increase in number in bacterial infections. They are phagocitic.
- Specific granules contain leuokocyte alkaline phosphatase (LAP), collagenase, lyzozyme, and lactoferrin.
- Azurophilic granules (lysosomes) contain proteinases, acid phosphatase, myeloperoxidase, and B-glucuronidase.
- Hypersegmented (6+ lobes) neutrophils are seen in vitamin B12 and folate deficiency.
- Increased band cells (immature neutrophils) reflect states of increased myeloid proliferation (during bacterial infections, CML).
- Chemotactic agents:
C5a, IL-8, LTB4, kallikrein, platelet-activating factor.

Lymphocytes
Smaller lymphocytes have very scant cytoplasm and a round nucleus with dense chromatin.
- About 10% of the lymphocytes are larger with more abundant cytoplasm and less condensed nuclear chromatin. A small number may have abundant cytoplasm and prominent azurophilic granules.
- Absolute lymphocyte c_hanges as children age_ . Through at least 2 years and even up to 8 years of age lymphocytes are the ***most abundant white cell in the peripheral blood. Too few lymphocytes is termed lymphopenia, and too many lymphocytes is lymphocytosis.

Monocytes
- These are normally the largest cells.
- Their nucleus is irregular and lobulated.
- Their ample cytoplasm is grayish - blue. Fine azurophilic granules can be seen.
- The cell outline is often irregular and the cytoplasm may also be vacuolated. The absolute monocyte count for children fluctuates somewhat with age . Too few monocytes is termed monocytopenia , and too many monocytes is monocytosis.
STEP:
- Found in blood, differentiate into macrophages in tissue.
- Have a large, KIDNEY-shaped nucleus. Extensive “frosted-glass” cytoplasm.

Eosinophils
- These are slightly larger than neutrophils with a bi-lobed nucleus.
- Their spherical granules are larger, coarse and reddish orange.
- The absolute eosinophil count remains fairly constant throughout life. Too many eosinophils is termed eosinophilia, ** while seeing none in a particular peripheral blood sample isn’t necessarily abnormal .
- STEP
- Defend against helminthic infections.*
- -Highly phagocytic for antigen-antobody complexes.*
- -Produce Histamine, major basic protein (MBP a helminthotoxin), eosinophil peroxidase, eosinophil cationic protein, and eosinophil-derived toxin.*

Basophils
Basophils are similar in size to neutrophils.
The nucleus is obscured by purple - black, coarse granules.
-These are the least abundant of all white cells in the peripheral blood . There is little variation in the absolute basophil count throughout life. Too many basophils is basophilia, while ***seeing none in a particular peripheral blood sample isn’t necessarily abnormal.
STEP
- Mediate allergic reactions
- Granules contain heparin (anticoagulant) and histamine (vasodilator).
- Synthesize and release leukotrienes.
Type I Helper T cells (Th1)
These helper T cells recognize antigens and make a lymphokine that attracts thousands of macrophages, the heavy-duty phagocytes, to the area where antigen has been recognized.
This helps to get rid of a serious infection, or a transplanted kidney.
Th17 Helper T cells
These are similar to Th1 in that their main role is to cause focused inflammation, although they are more powerful than Th1. They help resist some very tough infectious organisms, but they have been implicated in many serious forms of autoimmunity.
Type 2 Helper T cells, Th2
They stimulate macrophages to become ‘alternatively activated,’ and then function in walling - off pathogens and promoting healing, a process that usually takes place after the pathogen - killing Th1 response. They are very important in *parasite immunity*.
Follicular Helper T cells (Tfh)
After they are stimulated by antigen, they migrate from T cell areas of lymph nodes into the B cell follicles, where they help B cells get activated to make the IgM, IgG, IgE and IgA antibody subclasses.
Regularoty T cells (Treg)
Make lymphokines that suppress the activation and function of their sibling T helper cells , so they keep the immune response in check.
Th1 , Th2 , Th17, Tfh, and Treg have ► a molecular marker, called ___ , on their surface, whic h increases their affinity for antigen, helps get them activated, and also serves us as a convenient tag for their identification. ► CTL have a related marker, ___ .
CD4; CD8
Helpert T cells recognize pieces of antigens loaded onto____1____ APCs.
CTL recognize pieces of antigens loaded onto __2____ by APCs
1- MHC Class II, which is present in APCs.
2- MHC Class I, which is present in all cells.

