The definition of a battery
A device that converts chemical energy into electrical energy
Which type of battery cells are rechargeable and which aren’t?
Primary cell are not rechargeable Secondary cells are rechargable
Why are secondary cell batteries called “storage batteries”?
Secondary cells do not produce electrical energy. They store it in chemical form
Each cell in a Lead-acid battery produces how much voltage?
2.1V
The 2 main purposes for an aircraft battery
- To provide backup emergency electrical power 2. To provide power for starting an engine
Identify
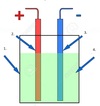
- Casing
- Annode
- Cathode
- Electrolyte
Identify

- Casing
- Positive terminal
- Negative terminal
- Cel divider
- Positive electrode (lead dioxide)
- Negative electrode (lead)
- Sulfuric acid and water
When a primary cell is fully charged, the potential differnce between the two electrodes is approximately
1.5V
In a closed circuit, as the transfer of electorns from the postive electrode to the negative electrode continues through the electrolyte, what eventally happens to the negative electrode in a primary cell battery?
It dissovles in the electrolyte
Oxidizing is the process of
loosing electrons
Identify (carbon-zinc)

- Metal cap
- Carbon rod
- Zinc Case
- Manganese Dioxide
- Ammonium chloride
- Metal bottom
The annodic, cathodic and electrolytic materials for an Alkaline battery
- zinc annode
- Manganese dioxide cathode
- Potassium hydroxide electrolyte
How can the composition of the electrolyte and electrodes and the chemical energy inside the electrolyte be restored?
Passing a charging current throught the battery in the reverse direction of discharge
The capacity of a battery cell is the measure of
How much current the battery can provide for a specific period of time
What must the charging voltage be while recharging a secondary cell battery?
Greater than the potential differnce between the electrodes
Capacity of a battery is measured in
Ampere hours
The determining factor of the capacity of a battery
Surface area of the electrodes (plates)
A cell with a capacity of 80 Ah should produce
80 amps for 1 hour
or
40 amps for 2 hours
or
8 amps for 10 hours
The 3 ways that battery cells can be connected in
- Serries
- Parallel
- Series-parallel
The total voltage in a battery where the cells are connected in series
The sum of the individual cell voltages
Desribe the total voltage and battery capacity of multiple battery cells arranged in series
the voltage will be the sum of the individual cells but the capacity will remain the same
Desribe the total voltage and battery capacity of multiple battery cells arranged in parallel
The total voltage will that of 1 cell, but the capacity will be the sum of the individual cell capacities
If two 12V, 80Ah batteries are connected in series, how will they act?
As a battery with 24 V output, 80 Ah
If two 12V, 80Ah batteries are connected in parallel, how will they act?
They will act as one battery with 12V out put, at 160Ah
The 2 type of secondary cell batteries used in aviation
- Lead acid
- Alkaline
The nominal voltage of each cell of a lead acid battery is
2 volts
Is the nominal voltage the same as the actual voltage
Most of the time, no buut close enough
How are the cells in a lead acid battery arranged?
In series
The annode of a lead acid battery is made of
lead peroxide
The cathode material in a lead acid battery is made of
spongey lead
The electrolyte composition of a lead acid battery is made of
sulfurice acid and water
Where the liquid level of the sulfuric acid electrolyte in a lead acid battery should be at
Just above the top of the plate
Why is there a need to replenish distilled water in a lead acid battery?
Water evaporated during normal use
When the cells of a lead-acid battery are working, what gas must be vented?
Hydrogen
The state of charge of a lead-acid cell can be deterimied by
Measuring the electrolyte souluiton
The instrument used to measure the SPG of the suluric acid solution in a lead-acid battery (and the formula)
Hydrometer ( SPG= weight/ weight of equal volume of distlled water)
A fully charged cell of a lead-acid battery will have a SPG value of
1.27
How does the acid in a lead-acid battery become weaker
When the battery is connected to an external circuit and current is flowing
Relationship between SPG of sulfuric acid electrolyte, and it’s acidity level
Proportional
A new fuly charged lead-acid battery will have ___% acid and ___%water
30% acid, 70% water
When the sulfuric acid electroltyte SPG falls below____, the battery is considered to be discharged
1.15
The standard refernce temperature for measuring the SPG of sulfuric acid electroylte with a hydrometer is:
80F
If a hydrometer test is done at a temperature other than 80F, what must be done?
Correction must be added using a correction chart
The lead peroxide in the positive electorde of a lead-acid battery turns into_________ during discharge
lead sulfate
What does the formed lead sulfate most noticably in a lead-acid battery durring use?
Increases internal resistance
Desribe the reason for not being able to recahrge a lead-acid battery if it’s been fully discharged a number of time
As it is discharged, lead sulfate builds up inbetween the cathode and annode plates
The two major aspects that the construction of a battery must compromise between
Energy vs power
(storing energy for long time use)
(being able to give a large poweroutput for a small period of time)
The four factors that affect the capacity of a lead-acid battery
- Amount of active material
- Surface area of the plates
- Amount of electrolyte
- Temperature
The rating used to specify the capacity of a battery
5- hour discharge rating
Description of the 5- hour discharge rating
Represents the number of Amp hours of capacity, when there is sufficient current flow, to reduce the voltage of the battery completly to zero, over the course of 5 hours
How to remove corosion off of battery terminals of a lead-acid battery (steps)
Gently scrub the box and terminals with a soft bristled brush and a solution of sodium bicarbonate.
Once clean, rinse with clean water and dry
Coat the battery terminals with petrolum jelly
Do you ever add acid to a lead-acid battery during servicing?
Only if it has spilled
Steps for receiving a new dry-charged state battery
- Pour in electrolyte (shipped with battery)
- Give a slow boost charge
- Let battery sit for an hour and adjust electroltye level
The sequence for mixing electrolyte solution for a lead-acid battery and why does it have to be in that sequence
must add the ACID to the WATER.
[If water is added to the acid, water is less dense and will float to the top of the acid which will cause a chemical reaction at the top of the container which boils the water and acid will splash outside of the container]
Should automotive and aircraft electorlytes be mixed?
No, differnce SPG’s
Relate the two to eachother: Battery internal resistance and charging current
The charging voltage needs to be higher than the open-circuit voltage of the battery to overcome the battery’s interal resistance
What is open-circuit voltage?
The voltage of the battery for EMF course when not connected to the circuit
The 2 methods of battery charging
- Constant current charging
- Constant voltage charging
synomn for open-circuit voltage
synonmn for no-load voltage
What is the onload or nominal voltage?
Voltage of a cell with load being used
What is the off load voltage?
Voltage with no load applied?
